
- Tel: +86 28 87436908
- Email: info@cdsentec.com
Pharmaceutical raw material process instrumentation plays a vital role in maintaining the quality and integrity of pharmaceutical raw materials throughout the production process.
Proper storage of pharmaceutical raw materials in pharmaceutical industry is essential to maintain their quality and integrity throughout the production process.
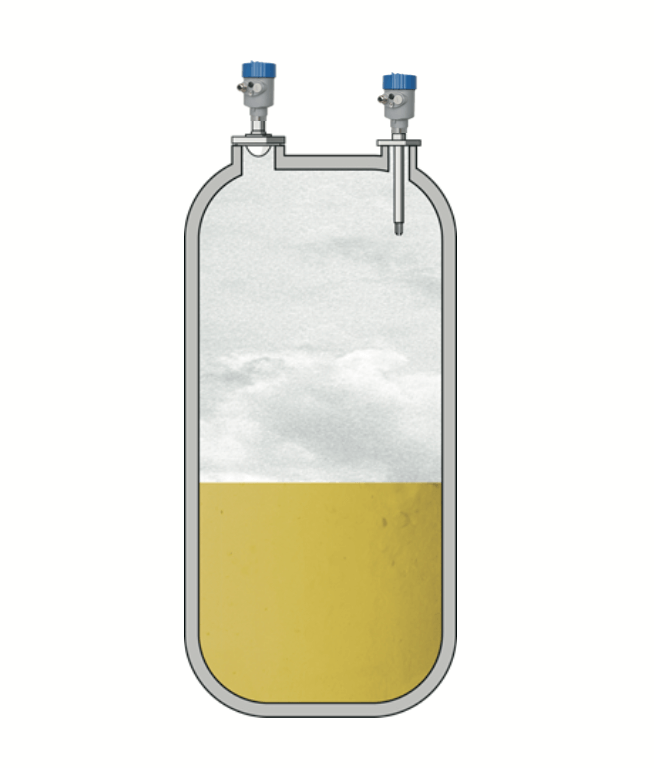
When producing products that are directly injected into the bloodstream or used as eye drops or nasal drops, it is necessary to use injection water with high purity. This water is produced through filtration and sterilization, and is temporarily stored in storage tanks. Absolute sterility and cleaning ability are the main requirements for all instrument components that come into direct contact with the medium. This also applies to measuring instruments for measuring liquid level and pressure in storage tanks.
Hexane is used as a solvent, reaction medium, and for extracting oils and fats. This colorless, slightly gasoline flavored liquid is prone to volatilization, and its vapor can form an explosive mixture with air. Therefore, no residue is allowed when emptying the storage tank. In order to ensure reliable storage, it is necessary to measure the material level.
Different solvents and carrier materials need to be prepared for subsequent production, which will then be added to the bioreactor and fermentation tank. The container is wrapped in a jacket heated by steam to control the temperature inside the storage tank. Control the filling and retrieval process by measuring the liquid level. Prevent tank overflow or idling by measuring the limit position.
In many production processes in the pharmaceutical industry, various small amounts of chemicals are required to improve the performance of specific products. In the production area, it is necessary to prepare these media directly in movable or transportable containers. Utilize the liquid level measurement function to ensure that liquid materials can be replenished for continuous process processes.
By accurately monitoring the liquid level and pressure of pharmaceutical raw material storage tank containers, pharmaceutical manufacturers can ensure the quality and safety of raw materials for producing high-quality drugs.
Pharmaceutical preparation involves the process of creating medications by combining various pharmaceutical raw materials. To ensure the integrity of the preparations and maintain their quality, proper storage environments are essential.
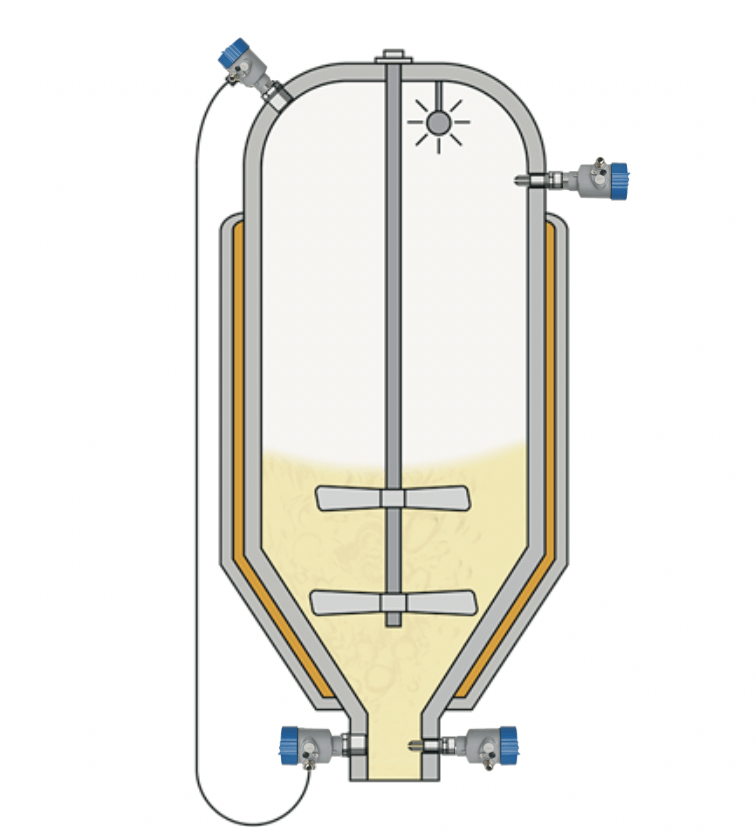
Many different ointments and creams are produced in batching tanks. The process conditions in batch production are typically characterized by high temperatures and vacuum. After each batch run, the tank needs to be cleaned quickly and effectively with chemically aggressive cleaning agents ready for the next batch. Contamination of the products during the mixing or reaction process is prevented by the use of a protective gas atmosphere. To ensure a safe, reliable process, both the level and the gas head pressure must be continuously monitored.
Mixing vessels are applied for preparation, storage and transfer of a wide range of pharmaceutical products. These vessels are used for the manufacture of antibiotics, blood plasma products, and injection and infusion solutions. Following preparation, substances are transferred to a filling station. To ensure a reliable process, the liquid level in the mixing vessel must be reliably and continuously monitored.
The various solvents and intermediate carrier materials have to be first prepared before they proceed onto further production steps. This is usually carried out in bioreactors and fermentation tanks. These vessels are enclosed with a steam heating jacket to regulate the temperature inside the mixing process. The level measuring system is needed to control filling and discharge as well as monitor during the process. Level detection prevents the tank from overfilling or running empty.
In the pharmaceutical industry, numerous liquids with widely different properties are required. Liquid intermediate products must be made available and held ready for further processes. To ensure smooth, continuous production, reliable level measurement and point level detection are essential.
Pharmaceutical preparation instrumentation refers to the equipment and devices used in the manufacturing and preparation of medications in the pharmaceutical industry. These instruments play a crucial role in ensuring the accuracy, precision, and safety of pharmaceutical preparations.
Pharmaceutical upstream refers to the initial stages of the pharmaceutical production process, which involve the sourcing, selection, and preparation of raw materials and components.
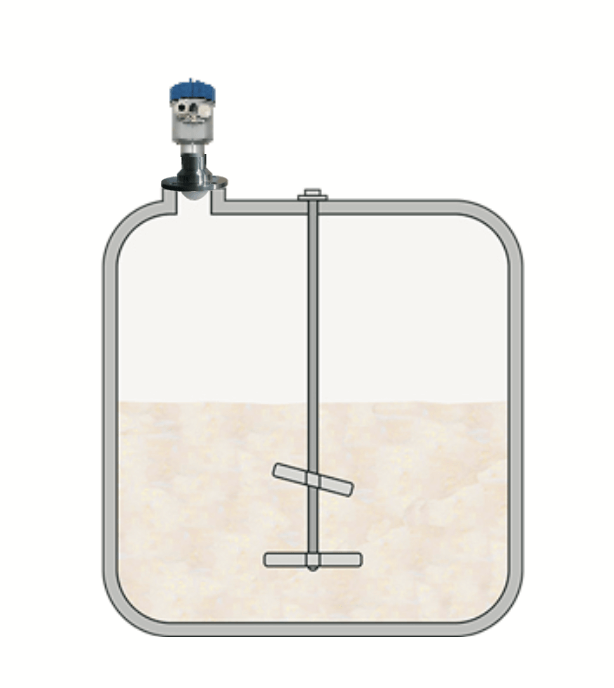
Some pharmaceutical companies use bioreactor vessels to cultivate particular cells or microorganisms required for certain treatments. The very high level of hygiene required means the vessels and all associated parts must be easy to thoroughly clean, because even the slightest contamination with other organisms can lead to an aborted production run. These reactors are mainly “batch-fed”, where they are completely filled at the start and not emptied again until the process is completed. The pressure and level in the reactor have to be monitored continuously during process in order to obtain a high-quality yield.
The measuring conditions inside a reaction vessel in a multi-product, multi-line production facility are characterized by changing media as well as widely fluctuating temperatures and pressures. Depending on the properties of the raw materials, either alloy, stainless steel or enamel lined vessels are used. Different components like stirrers, dryers and centrifuges also vary in each process. To ensure reliable operation and high productivity, both level and pressure have to be continuously measured and monitored.
When very small product batches are produced in the pharmaceutical industry, disposable plastic containers are often used. These are essentially polymer bags in which a fermentation process takes place or which contain a certain medium or buffer. Continuous level measurement is required to ensure high process efficiency.
Pharmaceutical upstream process instruments instruments are properly cleaned, maintained, and validated to prevent cross-contamination and ensure their proper functioning.
SenTec online resource library is a place you can know more knowledge from video, cases study, e-book etc.